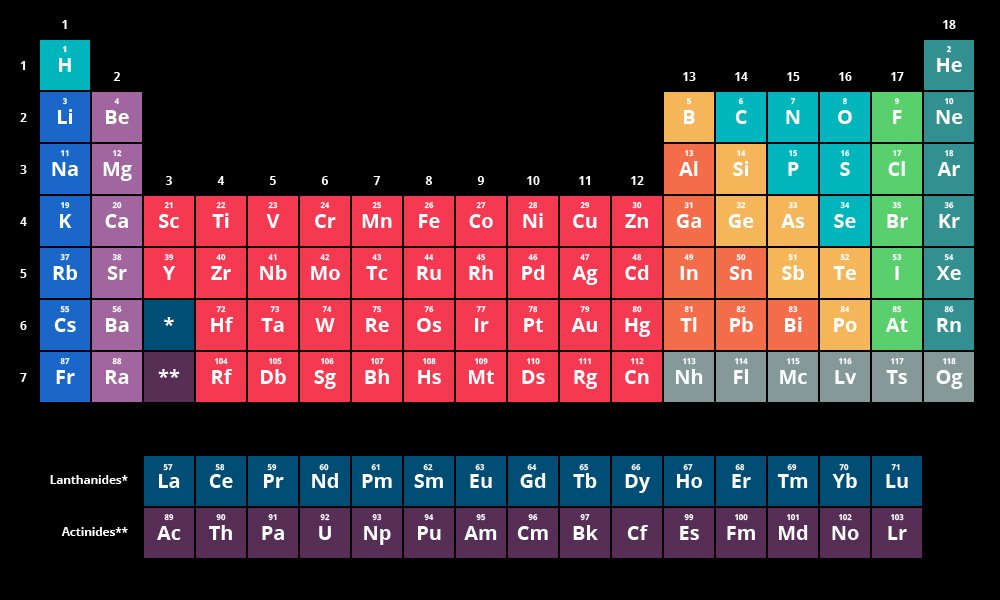
- The periodic table of chemical elements, often known as the periodic table, divides all known chemical elements into rows (called periods) and columns (called groups) based on increasing atomic number.
- The periodic table is used by scientists to quickly refer to information about an element, such as its atomic mass and chemical symbol.
- The design of the periodic table also allows scientists to detect patterns in element properties such as electronegativity, ionisation energy, and atomic radius.
- Many scientists worked on the problem of grouping the elements, but Dmitri Mendeleev is widely regarded as the periodic table’s inventor, having published his first version in 1869.
- Since then, the periodic table has changed to reflect almost 150 years of chemistry and physics scientific advancement and understanding. With 118 known elements today, it is often regarded as one of science’s most significant achievements.
What is the Periodic Table?
- The periodic table is an organisation of all known elements in order of increasing atomic number and recurring chemical properties.
- They are organised in a tabular format, with a row representing an era and a column representing a group.
- Elements are ordered in increasing atomic number order from left to right and top to bottom. Elements of the same group will therefore have the same valence electron configuration and, as a result, identical chemical characteristics.
- Elements in the same period, on the other hand, will have an increasing order of valence electrons.
- As a result, as the atom’s energy level rises, so does the number of energy sub-levels per energy level.
- The first 94 elements of the periodic table occur naturally, but the remaining elements from 95 to 118 have only been created in laboratories or nuclear reactors.
- The contemporary periodic table, which we presently use, is a revised and enhanced version of certain models proposed by scientists in the nineteenth and twentieth centuries.
Dimitri Mendeleev proposed his periodic table based on the discoveries of previous scientists such as John Newlands and Antoine-Laurent de Lavoisier. Mendeleev, on the other hand, is awarded sole credit for developing the periodic table.
Why Arrange Elements in a Table?
- The contemporary periodic table of chemical elements is as familiar as a map of the earth, yet it was not always so evident.
- Dmitri Mendeleev, the author of the periodic table, began collecting and organising known properties of elements while travelling by train in 1869, as if he were playing a game. He discovered groups of elements with similar features, but he also noticed that there were many exceptions to the emerging patterns.
- There were many critics, and it took years for Mendeleev’s patterns to be accepted internationally, but after freshly discovered elements matched those predicted by Mendeleev, his patterns could not be disregarded.
- Furthermore, some of the “fudged” attributes were later recalculated and found to be substantially closer to his predictions.
- Instead of giving up, he experimented with changing the measured property values to better fit the patterns! In order for the patterns in his “game” to operate, he anticipated that certain elements needed exist that did not exist at the time.
Who Created The Periodic Table?
- According to the Royal Society of Chemistry, Dmitri Mendeleev, a Russian chemist and inventor, is regarded as the “father” of the periodic table (opens in new tab).
Periodic Table: Mendeleev was a prominent lecturer at a university in St. Petersburg, Russia, in the 1860s. Mendeleev opted to produce one because there were no new organic chemistry textbooks in Russian at the time
Mendeleev Periodic Table
- Dimitri Mendeleev, usually regarded as the father of the periodic table, proposed the initial iteration of the periodic table, which is still in use today.
- Mendeleev’s periodic law differs from contemporary periodic law in one important way.
- Mendeleev based his periodic table on increasing atomic mass, but modern periodic law is based on increasing atomic number order.
- Mendeleev’s periodic chart, despite being based on atomic weight, was able to predict the discovery and qualities of certain elements.
- Only roughly half of the elements known to us now were known during his time, and much of the information known about the elements was incorrect. Mendeleev’s Periodic Table was first published in 1869 in the German Journal of Chemistry.
Periodic Table: FAQs
1.In chemistry, what is a periodic table?
Answer. The periodic table of chemical elements, often known as the periodic table, divides all known chemical elements into rows (called periods) and columns (called groups) based on increasing atomic number.
2.What is the definition of the periodic table of elements?
Answer. A chart of chemical elements that shows them in rows horizontally in increasing atomic number order and vertically in similarity of chemical properties of their atoms.
3.How many different components are there?
Answer. The Periodic Table has 118 elements.
4.What is the Gold Symbol?
Answer. Au is the symbol of Gold.
5.Iodine’s Discoverer
Answer. Bernard Courtois discovered iodine in 1811.
6.What is the Silver Symbol?
Answer. Ag is the symbol of Silver.
7.What are the 118 elements known as?
Answer. Nihonium, moscovium, tennessine, and oganesson are the permanent names for elements 113, 115, 117, and 118.
8.What is on the periodic table?
Answer. Tennessine is a chemical element that has the symbol Ts and the atomic number 117.
9.What is the most powerful element?
Answer. Oganesson, named after Russian physicist Yuri Oganessian is the heaviest element on the periodic table at the moment.
