The combining capacity of atoms or molecules is measured in valency. As a result, it is the ability of a single element’s atom to react and combine with specific numbers of atoms from another element. The combining capacity of an element with other atoms when it forms chemical compounds of the molecule is measured by its valence or valency.
Electrons in an atom are organised into orbitals (shells) designated by letters such as K, L, M, N, and so on. The electrons in an atom’s outermost shell/orbit are known as valence electrons. Because the outermost orbit usually contains more energy than the electrons in other orbits, valence electrons participate in any chemical reaction.
According to the Bohr-bury scheme, an atom’s outermost orbit can have up to 8 electrons. When the outermost orbit is completely filled, however, the element exhibits very little to no chemical activity. Their ability to combine becomes insignificant or non-existent.
Achieving Complete Octet
When an atom’s outermost shell has a total of 8 electrons, the atom is said to have completed an octet. To obtain a complete octet, an atom must gain, lose, or share a specific number of electrons from its outermost orbit. As a result, an atom’s capacity is the total number of electrons gained, lost, or shared in the outermost atom to complete its octet arrangement. The valency of an atom is also determined by its capacity.
Because their outermost orbit is completely filled, noble gases are the least reactive. Other elements’ reactivity, on the other hand, is determined by their ability to form noble gas configurations. It will also aid in determining an atom’s valency.
For example, because hydrogen has one electron in its outermost orbit, it must lose one electron to achieve octet stability. As a result, hydrogen has a valency of 1. Similarly, magnesium has two electrons in its outermost orbit that it must lose in order to achieve octet and stability. As a result, magnesium has a valency of 2.
Examples of Valency
Valency of Sodium
Sodium has the atomic number 11 (Z=11). 2, 8, 1 is the electronic configuration of sodium. In the shells K, L, and M, 2, 8, 1 electrons are distributed. As a result, sodium has one valence electron and must lose one electron from the outermost orbit to achieve octet. As a result, sodium has a valency of 1.

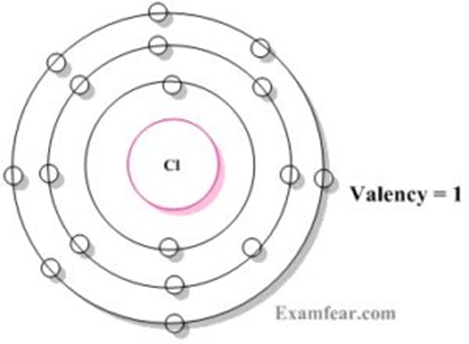
Valency of Chlorine
Chlorine has the atomic number 17 (Z=17). Chlorine’s electronic configuration is written as 2, 8, 7. In the shells K, L, and M, 2, 8, 7 electrons are distributed. As a result, chlorine’s valence electron is 7, and it needs to gain one electron from the outermost orbit to reach octet. As a result, chlorine has a valency of 1.
Methods of Determining Valency
1)The Octet Rule
If the periodic table cannot be used to determine valency, the octet rule is used. This rule states that atoms of an element or chemical have a tendency to gain or lose 8 electrons in their outermost orbit in whatever form of compound they are present in. In its outermost orbit, an atom can have up to 8 electrons. An atom’s stability is determined by the presence of 8 electrons in its outermost shell.
If an atom has one to four electrons in its outermost orbit, it is more likely to lose them. Positive valency is achieved when an atom donates its free electrons. If an atom has four to seven electrons in its outermost orbit, it will gain electrons. In such cases, accepting rather than donating an electron is preferable. As a result, the valency is calculated by subtracting the number of electrons from 8. Except for helium, all noble gases have eight electrons in their outermost orbit. In its outermost orbit, helium has two electrons.
2) Using the Periodic Table
The periodic table chart is used to calculate valency in this method. For example, all metals in column 1 have valency +1, including hydrogen, lithium, sodium, and so on. Similarly, all of the elements in column 17 have a valency of -1, such as fluorine and chlorine. Column 18 contains all of the noble gases. These elements have a valency of 0 and are inert.
There is, however, one exception to this valency determination method. Copper, iron, and gold, for example, have multiple active shells. This difference is most noticeable in transitional metals from column 3 to 10. It’s also found in heavier elements like lanthanides (57-71) and actinides (columns 11-14).